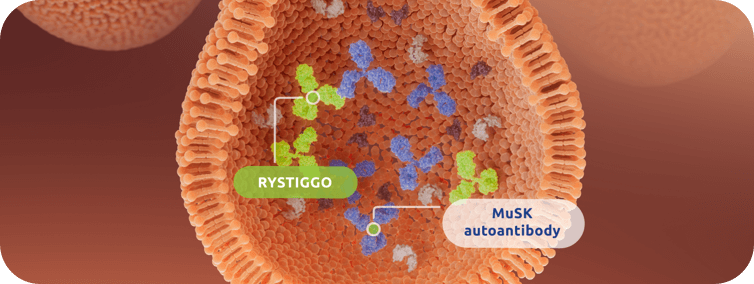
RYSTIGGO is the first FDA-approved targeted treatment for adults with either anti-AChR
antibody-positive or anti-MuSK antibody-positive generalized myasthenia gravis (gMG)1
POWERFUL EFFICACY
IN ACTION1
Actor portrayal.
POWERFUL
Statistically significant reduction vs placebo in MG-ADL total score (-3.4 vs -0.8; P<0.001) along with clinically meaningful (≥2.0-point) improvement at Week 6 in the pivotal study1,2*
The efficacy of RYSTIGGO was established in an up to 18-week, multicenter, randomized, double-blind, placebo-controlled study of 200 patients.1
CONSISTENT
Similar reduction in MG-ADL total score (primary endpoint in pivotal study) across the pivotal study and subsequent treatment cycles in the extension studies1,3†‡
The RYSTIGGO extension studies were open label and not placebo controlled; therefore, the efficacy and clinical significance of the results should be interpreted with caution.
Adults from the RYSTIGGO pivotal study could enroll in two extension studies that further evaluated the safety and efficacy of RYSTIGGO over time. The primary endpoints in the extension studies evaluated the proportion of patients experiencing TEAEs.3-5
There was a clinically meaningful (≥2.0-point) improvement in MG-ADL total score from baseline to Week 6 (Day 43) and across subsequent cycles in the extension studies.2,3
FLEXIBLE
Individualized break between treatment cycles based on clinical evaluation1§
RYSTIGGO is administered once weekly in 6-week dosing cycles followed by a treatment break determined by clinical evaluation.1
Actor portrayal.
First FDA-approved targeted treatment
for adults with anti-MuSK Ab+ gMG; commercially available for 2+ years1
Minimal Symptom Expression (MSE) was achieved
- MG-ADL score of 0 or 1 - in adults taking RYSTIGGO in the pivotal study (RYSTIGGO 7 mg/kg [n=66]: 25.8%, 10 mg/kg [n=67]: 28.4%, placebo [n=67]: 3.0%)2∥
Flexible dosing with individualized treatment breaks between cycles
- 6 weekly subcutaneous infusions followed by a treatment break determined by clinical evaluation1¶#
MSE was an exploratory endpoint not controlled for multiplicity. Therefore, data should be interpreted with caution and conclusions cannot be drawn.
The efficacy and safety of RYSTIGGO for the treatment of adults with anti-AChR Ab+ and anti-MuSK Ab+ gMG was established in an up to 18-week, multicenter, randomized, double-blind, placebo-controlled Phase 3 study. In the study, 200 adults with gMG at least 18 years of age were randomized 1:1:1 to receive weight-tiered doses of RYSTIGGO (n=133), either 7 mg/kg (n=66) or 10 mg/kg (n=67) of RYSTIGGO, or placebo (n=67).1
Adults who reached MSE achieved an MG-ADL score of 0 or 1 at any time up to and including Week 6 (Day 43) of the pivotal study.2
RYSTIGGO is intended for subcutaneous administration using an infusion pump at a constant flow rate of up to 20 mL/hr.1
If a scheduled infusion is missed, RYSTIGGO may be administered up to 4 days after the scheduled time point. Thereafter, resume the original dosing schedule until the treatment cycle is completed.1
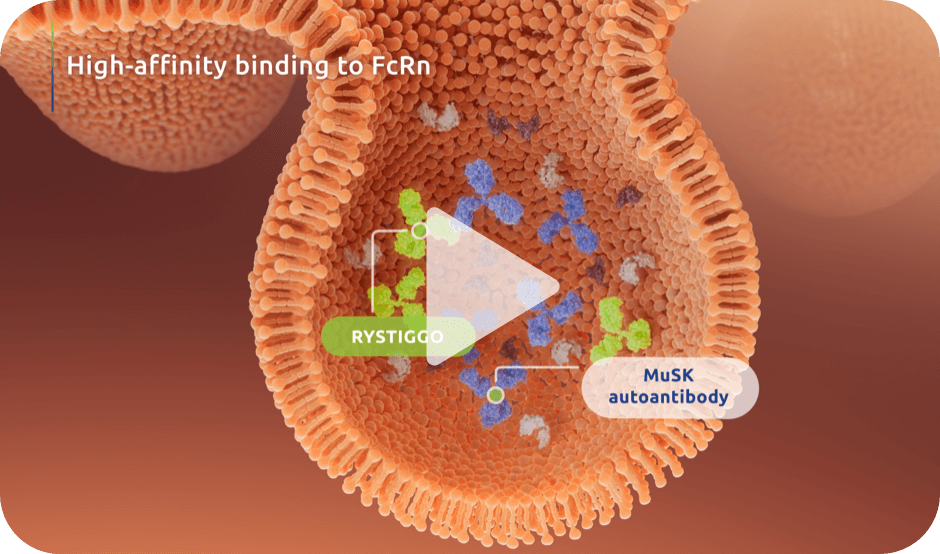
Watch the RYSTIGGO Mechanism of Action Video
Explore how RYSTIGGO binds to FcRn with high affinity, reducing IgG recycling and leading to degradation.1,6**††
The precise mechanism through which RYSTIGGO exerts therapeutic effects is unknown.
Based on in vitro data.6


Start Your Patients on RYSTIGGO
Learn how to enroll patients in ONWARD®, a personalized support program built to help patients through every step of their RYSTIGGO treatment journey.
Access Coding and Billing Support Materials
Download a helpful reference guide with important codes, including J-Code J9333, sample claim forms, and coding and billing information for RYSTIGGO.
Let's stay connected
Stay Up to Date on RYSTIGGO
Sign up to receive more information and updates about RYSTIGGO.
Contact a Sales Representative
Request a sales representative to contact you for more information about RYSTIGGO and schedule an office visit.
Ab+=antibody positive; AChR=acetylcholine receptor; FcRn=neonatal Fc receptor; MG-ADL=Myasthenia Gravis Activities of Daily Living; MuSK=muscle-specific tyrosine kinase; TEAEs=treatment-emergent adverse events.
References:
- RYSTIGGO [Prescribing Information]. Smyrna, GA: UCB, Inc.
- Bril V, Drużdż A, Grosskreutz J, et al. Safety and efficacy of rozanolixizumab in patients with generalised myasthenia gravis (MycarinG): a randomised, double-blind, placebo-controlled, adaptive phase 3 study. Lancet Neurol. 2023;22(5):383-394. doi:10.1016/S1474-4422(23)00077-7
- Bril V, Drużdż A, Grosskreutz J, et al. Rozanolixizumab in generalized myasthenia gravis: pooled analysis of the phase 3 MycarinG study and two open-label extensions. J Neuromuscul Dis. 2025;12(2):218-230. doi:10.1177/22143602241305511
- A study to investigate the long-term safety, tolerability, and efficacy of rozanolixizumab in adult patients with generalized myasthenia gravis. ClinicalTrials.gov identifier: NCT04124965. Updated September 5, 2023. Accessed February 27, 2025. https://clinicaltrials.gov/study/NCT04124965
- A study to evaluate rozanolixizumab in study participants with generalized myasthenia gravis. ClinicalTrials.gov identifier: NCT04650854. Updated February 26, 2024. Accessed February 27, 2025. https://clinicaltrials.gov/study/NCT04650854
- Smith B, Kiessling A, Lledo-Garcia R, et al. Generation and characterization of a high affinity anti-human FcRn antibody, rozanolixizumab, and the effects of different molecular formats on the reduction of plasma IgG concentration. MAbs. 2018;10(7):1111-1130. doi:10.1080/19420862.2018.1505464