EFFICACY
Rapid efficacy in action for a broad population of adults with gMG1,2*
In a clinical study, RYSTIGGO demonstrated statistically significant improvements vs placebo in MG-ADL total score at Week 6 (-3.4 vs. -0.8; P<0.001), with improvements observed as early as Week 1.1
*The clinical study included adults who were anti-AChR Ab+ and anti-MuSK Ab+, and who had MGFA Class II-IVa disease, an MG-ADL score of ≥3 (with ≥3 points from non-ocular symptoms), serum immunoglobulin G levels ≥5.5 gL, and who were on a stable dose of MG therapy prior to screening.1
Rapid efficacy in action for a broad population of adults with gMG1,2*
In a clinical study, RYSTIGGO demonstrated statistically significant improvements vs placebo in MG-ADL total score at Week 6 (-3.4 vs. -0.8; P<0.001), with improvements observed as early as Week 1.1
*The clinical study included adults who were anti-AChR Ab+ and anti-MuSK Ab+, and who had MGFA Class II-IVa disease, an MG-ADL score of ≥3 (with ≥3 points from non-ocular symptoms), serum immunoglobulin G levels ≥5.5 gL, and who were on a stable dose of MG therapy prior to screening.1
PRIMARY ENDPOINT: MG-ADL
Adults taking RYSTIGGO experienced rapid, clinically meaningful, and statistically significant improvements in activities of daily living in a 6-week treatment cycle1,2†
Change from baseline to Week 6 (Day 43) in MG-ADL total score in adults who are anti-AChR Ab+ or anti-MuSK Ab+1-3‡


†Clinically meaningful was established as a ≥2-point improvement in MG-ADL total score.2
‡During the treatment period, RYSTIGGO or placebo were administered subcutaneously once a week for 6 weeks.1
§Treatment initiation on Day 1.2
Adults taking RYSTIGGO achieved maximum efficacy at Week 6 (Day 43).1
The efficacy of RYSTIGGO for the treatment of adults with anti-AChR Ab+ and anti-MuSK Ab+ gMG was established in an up to 18-week, multicenter, randomized, double-blind, placebo-controlled study. In the study, 200 patients were randomized 1:1:1 to receive weight-tiered doses of RYSTIGGO (n=133), either 7 mg/kg of RYSTIGGO (n=66) or 10 mg/kg of RYSTIGGO (n=67), or placebo (n=67). The study included a 4-week screening period and a 6-week treatment period followed by 8 weeks of observation.1
MG-ADL assesses the impact of gMG on daily functions of 8 symptoms on a scale of 0-3, with total scores ranging from 0-24. Higher scores are interpreted as greater impairments. An improvement of ≥2 points was established as clinically meaningful.1,2
Measures include4:
- Voice/speech problems
- Chewing
- Swallowing
- Breathing
- Brushing teeth and/or combing hair
- Rising from a chair
- Diplopia
- Eyelid droop
EXPLORATORY SECONDARY ENDPOINT: MG-ADL Responder Rate
Proportion of MG-ADL responders from baseline at Week 6 (Day 43)2
MG-ADL responders: ≥2.0-point improvement in MG-ADL total score from baseline at Week 6 (Day 43)2
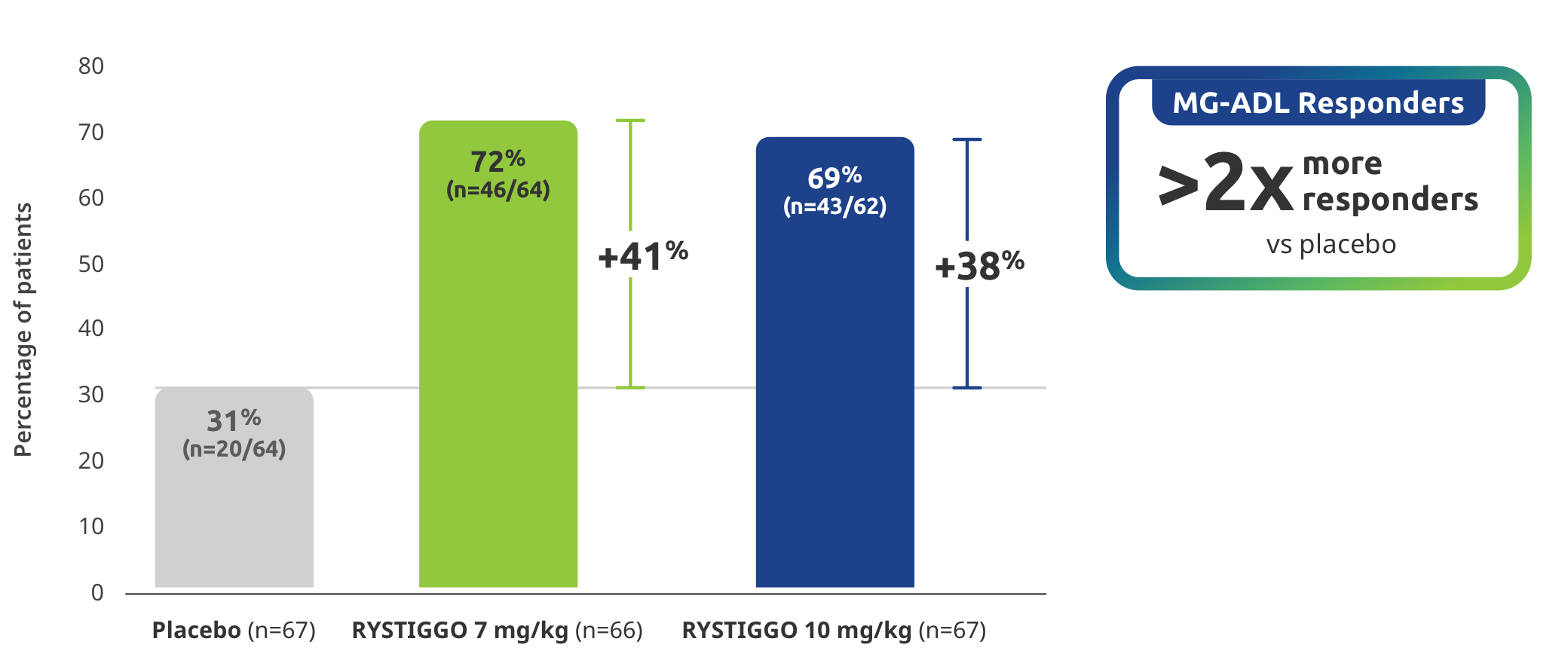

Study limitations: MG-ADL Responder Rate was a prespecified secondary endpoint not controlled for multiplicity; therefore, data should be interpreted with caution and conclusions cannot be drawn.
SUBGROUP ANALYSIS: MG-ADL IN ANTI-MuSK Ab+ PATIENTS
The first and only treatment indicated for adults with anti-MuSK Ab+ gMG1
Subgroup analysis of the primary efficacy endpoint: Mean change from baseline to Week 6 (Day 43) in MG-ADL total score in adults with anti–MuSK Ab+ gMG2,5
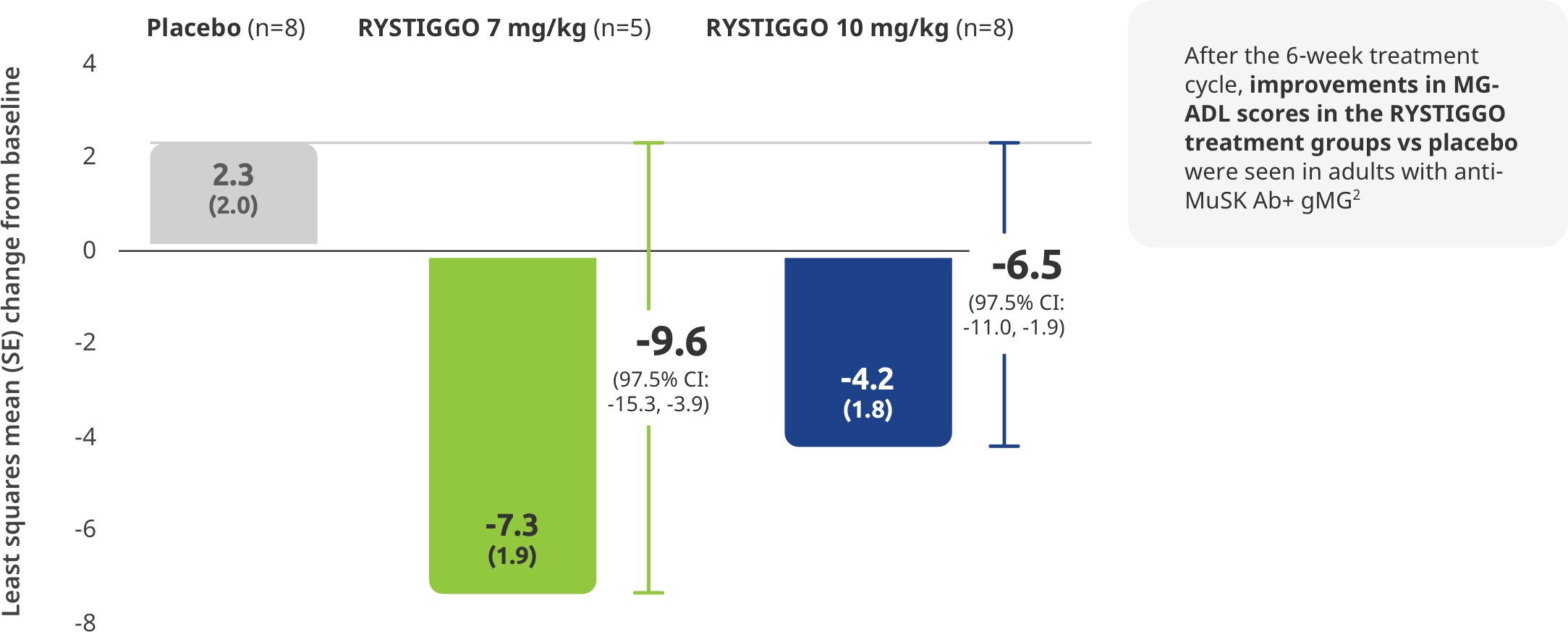
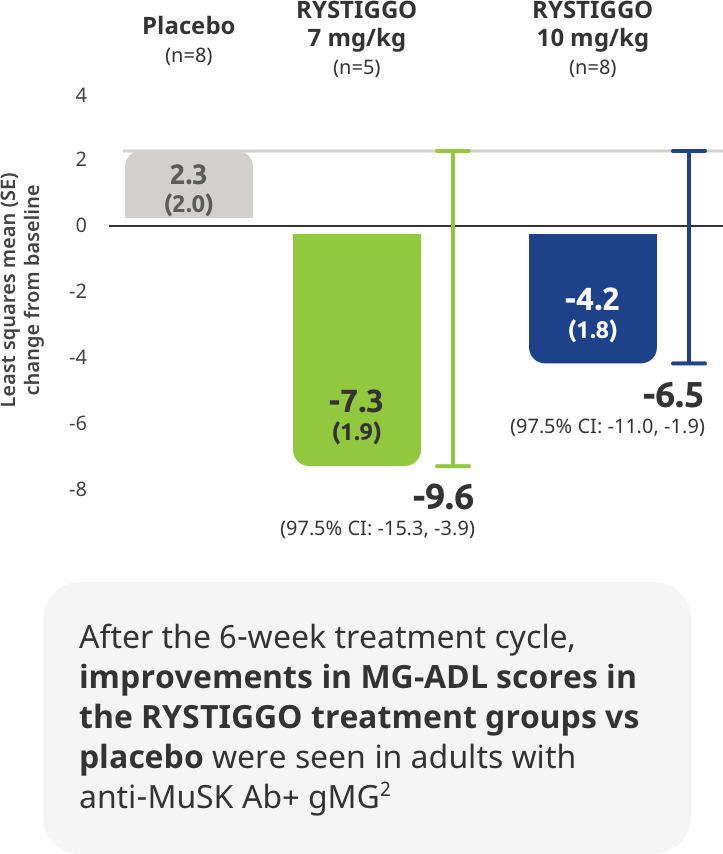
All adults with anti-MuSK Ab+ gMG who received RYSTIGGO and had data available at Week 6 (Day 43) were MG-ADL responders2
Subgroup analysis of MG-ADL responder rate: ≥2.0-point improvement in MG-ADL total score from baseline at Week 6 (Day 43) in adults with anti-MuSK Ab+ gMG2
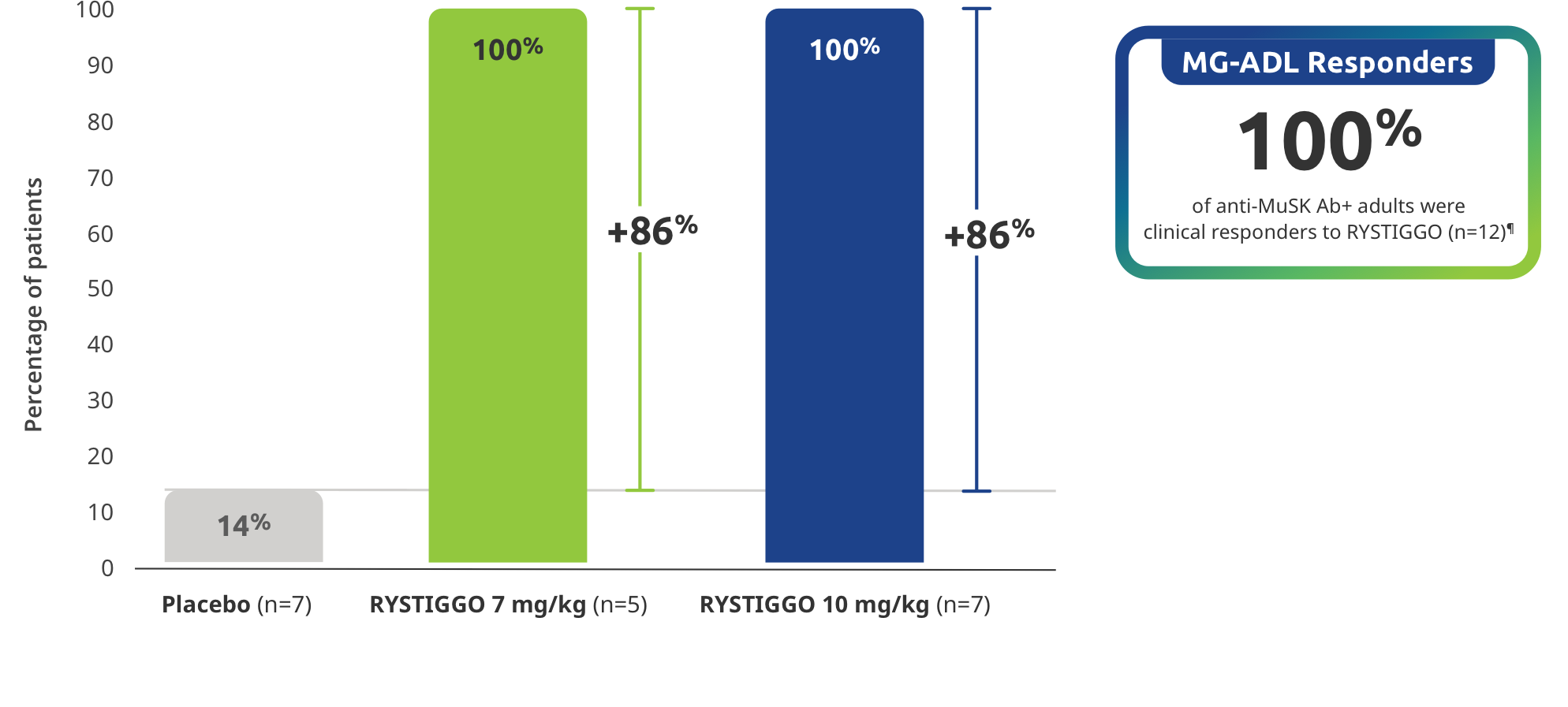

¶A clinical responder was established as a patient with a ≥2-point improvement in the total MG-ADL score compared to baseline at Week 6 (Day 43). Clinical responder data were not collected for one patient in the RYSTIGGO 10 mg/kg group who discontinued treatment.2,3
Study limitations: MG-ADL Responder Rate was a prespecified secondary endpoint not controlled for multiplicity; therefore, data should be interpreted with caution and conclusions cannot be drawn.
OTHER MG-ADL EFFICACY ENDPOINT
Minimal Symptom Expression (MSE) rates observed during the pivotal study2
Other efficacy endpoint: Proportion of adults achieving MSE during treatment and observation in the pivotal study2
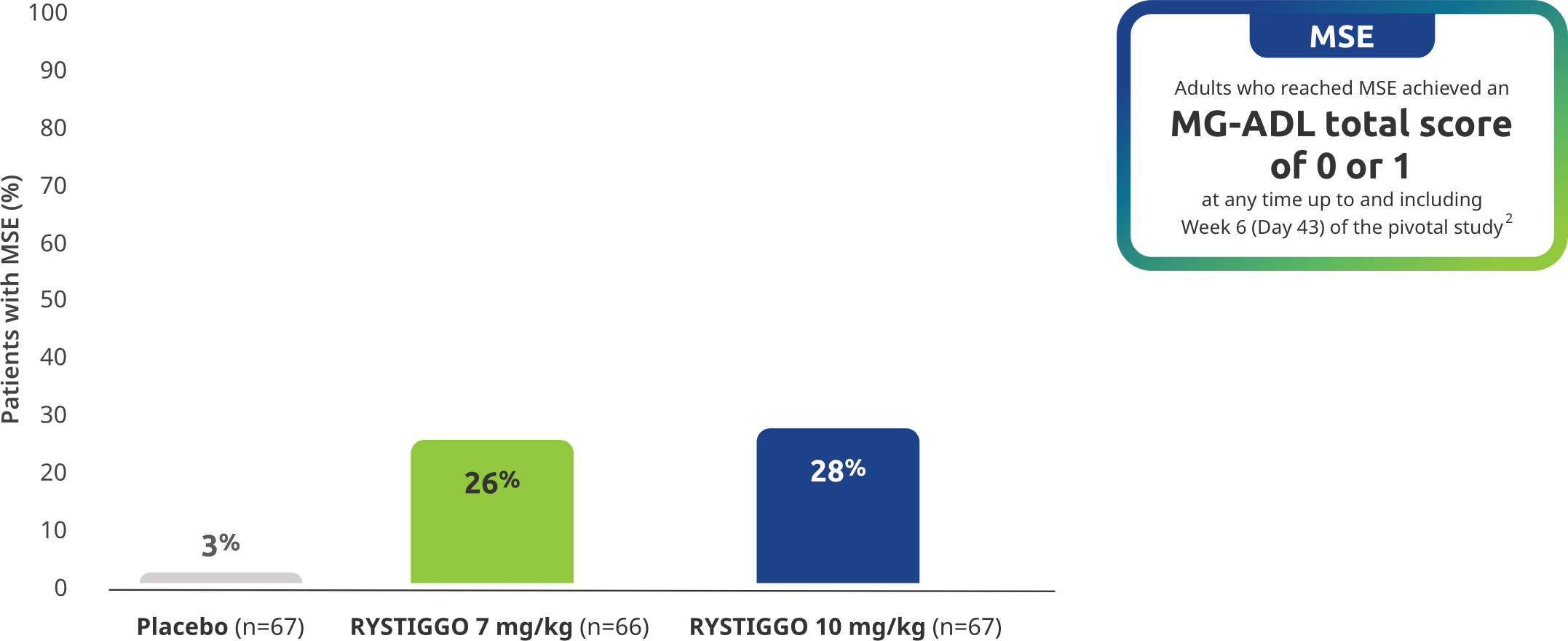

MSE was an exploratory endpoint and not controlled for multiplicity. Therefore, data should be interpreted with caution and conclusions cannot be drawn.


Review the RYSTIGGO MG-ADL results and MSE rates with a gMG expert
Ab+=antibody positive; AChR=acetylcholine receptor; CI=confidence interval; gMG=generalized myasthenia gravis; LS=least squares; MG-ADL=Myasthenia Gravis Activities of Daily Living; MG=myasthenia gravis; MGFA=Myasthenia Gravis Foundation of America; MuSK=muscle-specific tyrosine kinase; SE=standard error.
References:
- RYSTIGGO [Prescribing Information]. Smyrna, GA: UCB, Inc.
- Bril V, Drużdż A, Grosskreutz J, et al. Safety and efficacy of rozanolixizumab in patients with generalised myasthenia gravis (MycarinG): a randomised, double-blind, placebo-controlled, adaptive phase 3 study. Lancet Neurol. 2023;22(5):383-394. doi:10.1016/S1474-4422(23)00077-7
- Data on file. UCB Inc., Smyrna, GA.
- MG activities of daily living (MG-ADL) profile. Myasthenia Gravis Foundation of America. 1997. Accessed May 13, 2024. https://myasthenia.org/Portals/0/ADL.pdf
- Habib A, Kaminski H, Drużdż A, et al. Efficacy of rozanolixizumab in muscle specific kinase antibody-positive generalized myasthenia gravis: outcomes from the randomized, phase 3 MycarinG study. Paper presented at: AANEM 2022; September 21-24, 2022; Nashville, TN.
The efficacy of RYSTIGGO for the treatment of adults with anti-AChR Ab+ and anti-MuSK Ab+ gMG was established in an up to 18-week, multicenter, randomized, double-blind, placebo-controlled study. In the study, 200 patients were randomized 1:1:1 to receive weight-tiered doses of RYSTIGGO (n=133), either 7 mg/kg of RYSTIGGO (n=66) or 10 mg/kg of RYSTIGGO (n=67), or placebo (n=67).1
QUANTITATIVE MYASTHENIA GRAVIS (QMG)
Adults taking RYSTIGGO experienced statistically significant improvements in QMG score vs placebo1
Secondary endpoint: Mean change in QMG score from baseline to Week 6 (Day 43)1


†Improvements of ≥3 points were established as clinically meaningful.2
QMG is a physician assessment scoring system that quantifies disease severity. Measures are assessed on a scale of 0-3, with total scores ranging from 0-39. An improvement of ≥3 points was established as clinically meaningful.1-3
Measures include3:
- Ptosis
- Facial muscle weakness
- Dysarthria
- Grip strength
- Neck flexion endurance
- Diplopia
- Difficulty swallowing a cup of water
- Percent predicted forced vital capacity
- Arm and leg endurance
MYASTHENIA GRAVIS COMPOSITE (MGC)
Adults taking RYSTIGGO experienced statistically significant improvements in MGC score vs placebo2,4
Secondary endpoint: Mean change in MGC score from baseline to Week 6 (Day 43)2,4


‡Improvements of ≥3 points were established as clinically meaningful.2
MGC is a 10-item patient and physician assessment of the signs and symptoms of myasthenia gravis based on exam and patient history, with total scores ranging from 0-50. Higher scores are interpreted as greater impairments. An improvement of ≥3 points was established as clinically meaningful.2,5,6
Measures include5:
- Ptosis
- Eye closure
- Chewing
- Breathing
- Shoulder abduction
- Diplopia
- Talking
- Swallowing
- Neck flexion or extension
- Hip flexion
MYASTHENIA GRAVIS SYMPTOMS PATIENT-REPORTED OUTCOME (MG SYMPTOMS PRO)
MG Symptoms PRO evaluated gMG symptoms across 3 domains2:
Improvement in muscle weakness fatigability demonstrated in adults taking RYSTIGGO2,4
Secondary endpoint: Mean change in MG Symptoms PRO muscle weakness fatigability score from baseline to Week 6 (Day 43)2,4
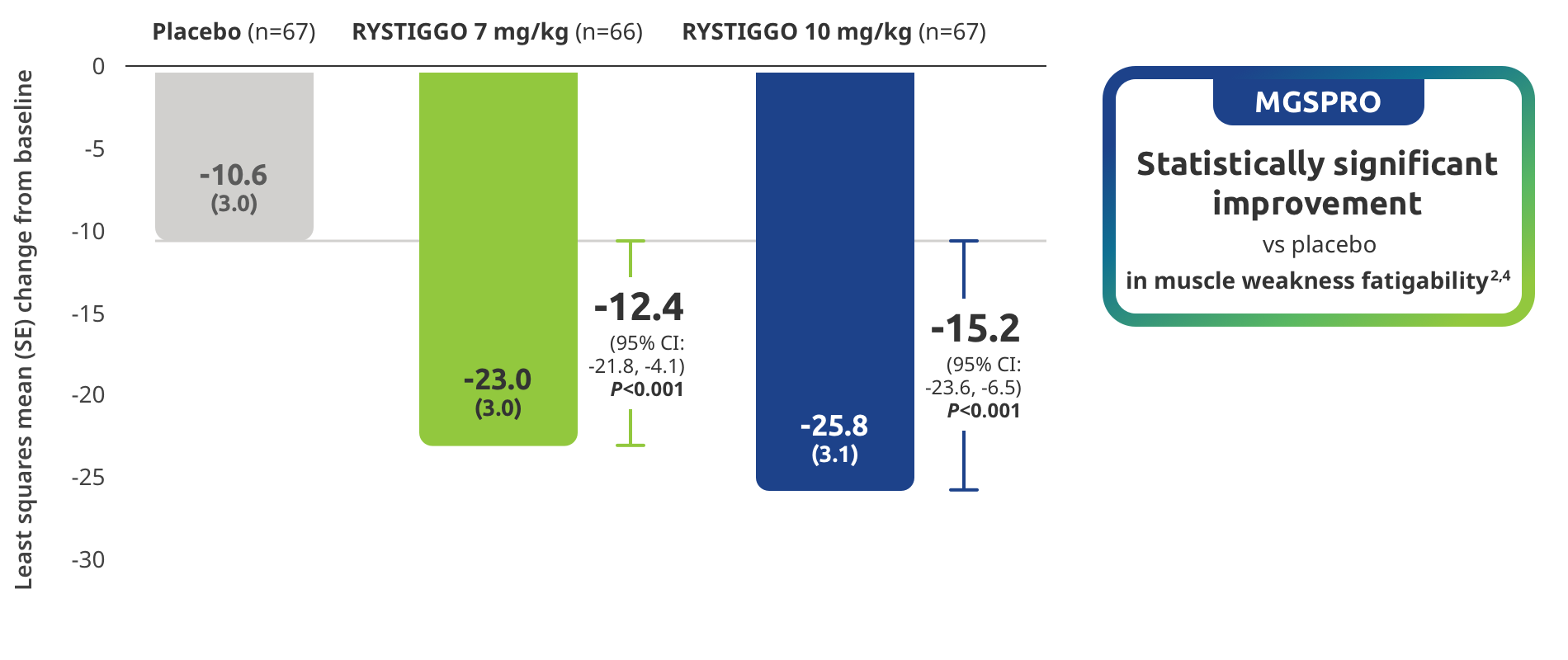
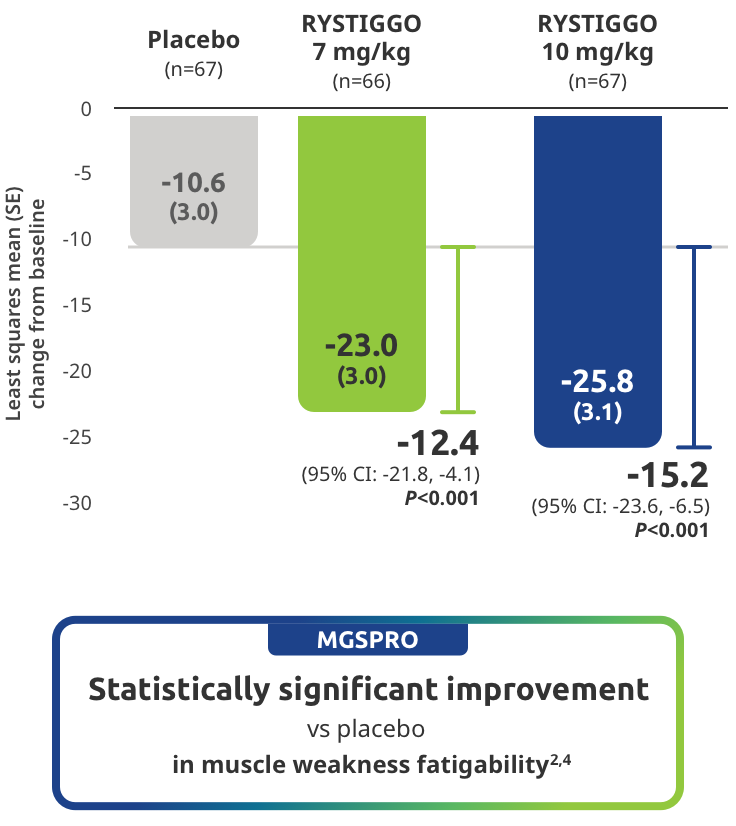
MG Symptoms PRO was part of the planned efficacy analysis; however, efficacy or clinical significance should be interpreted with caution.
Improvement in physical fatigue demonstrated in adults taking RYSTIGGO2,4
Secondary endpoint: Mean change in MG Symptoms PRO physical fatigue score from baseline to Week 6 (Day 43)4
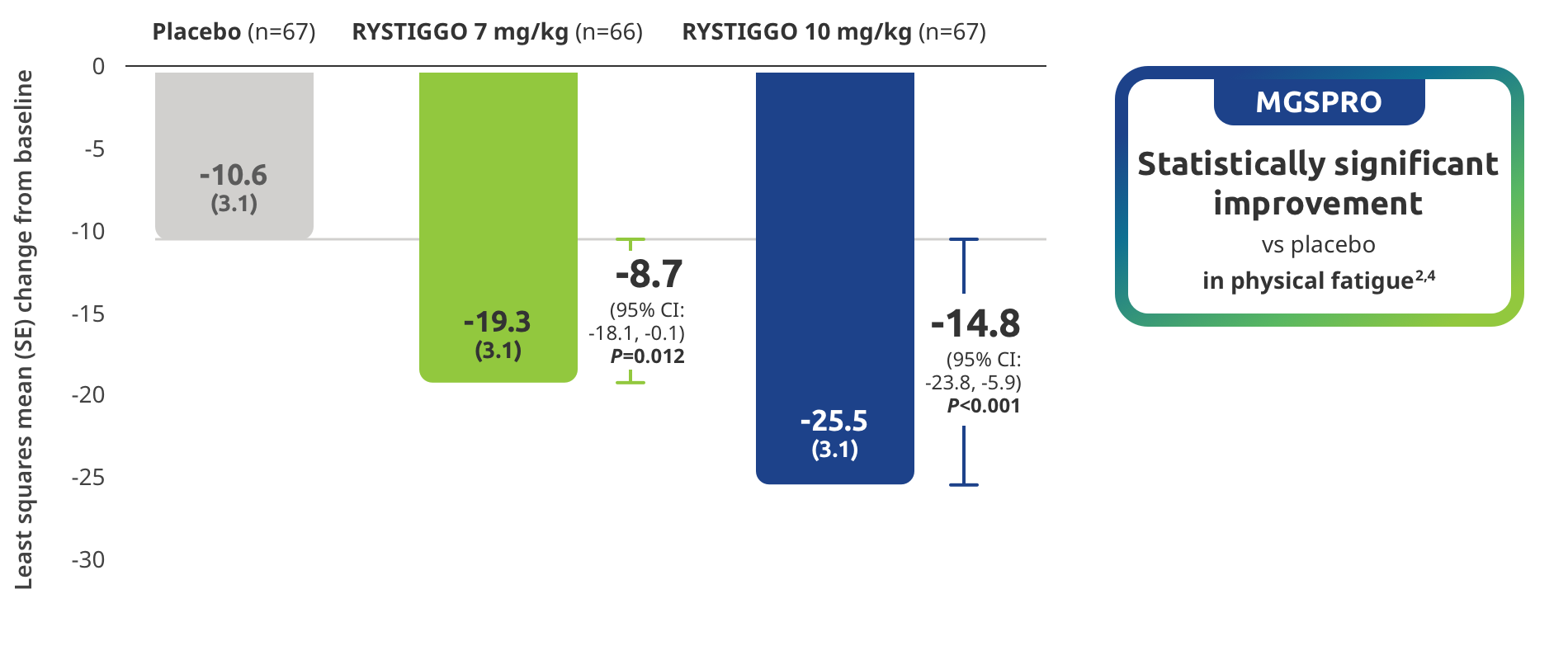

MG Symptoms PRO was part of the planned efficacy analysis; however, efficacy or clinical significance should be interpreted with caution.
Improvement in bulbar muscle weakness demonstrated in adults taking RYSTIGGO2,4
Secondary endpoint: Mean change in MG Symptoms PRO bulbar muscle weakness score from baseline to Week 6 (Day 43)4
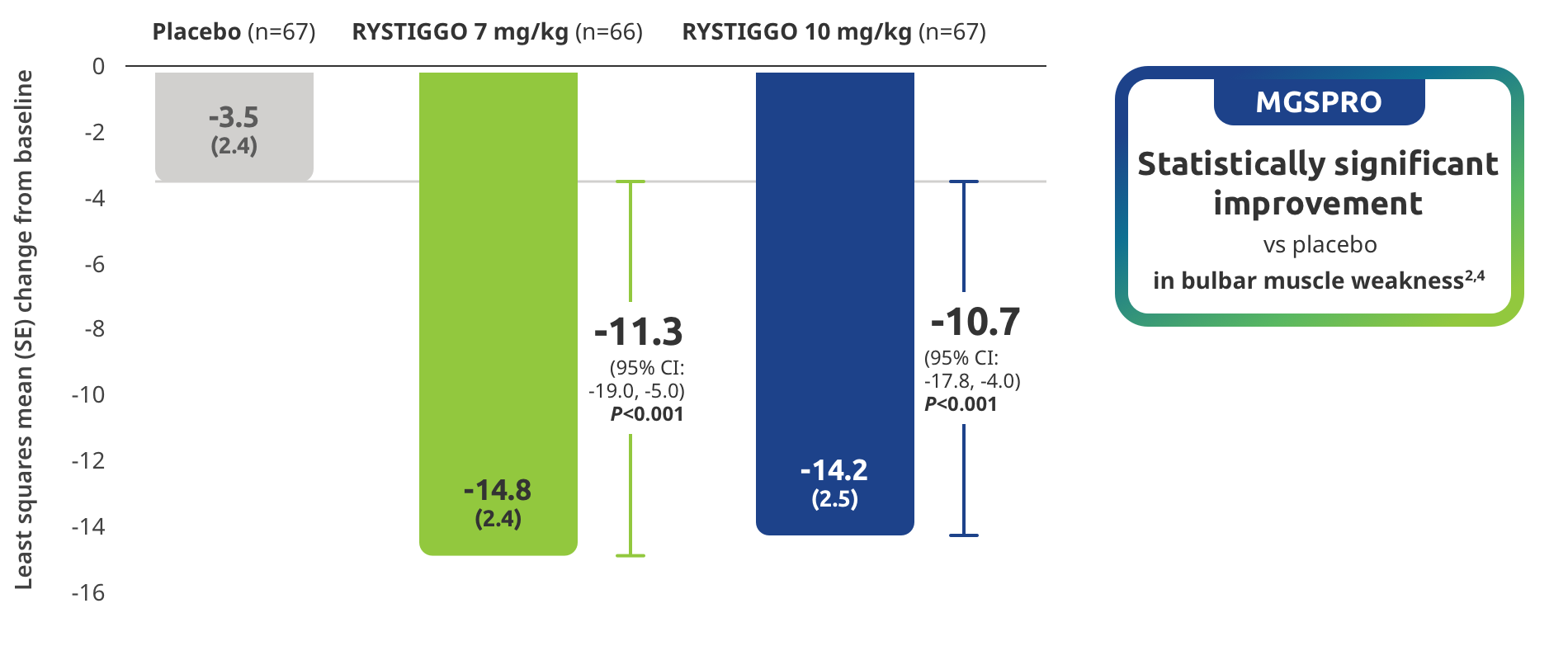
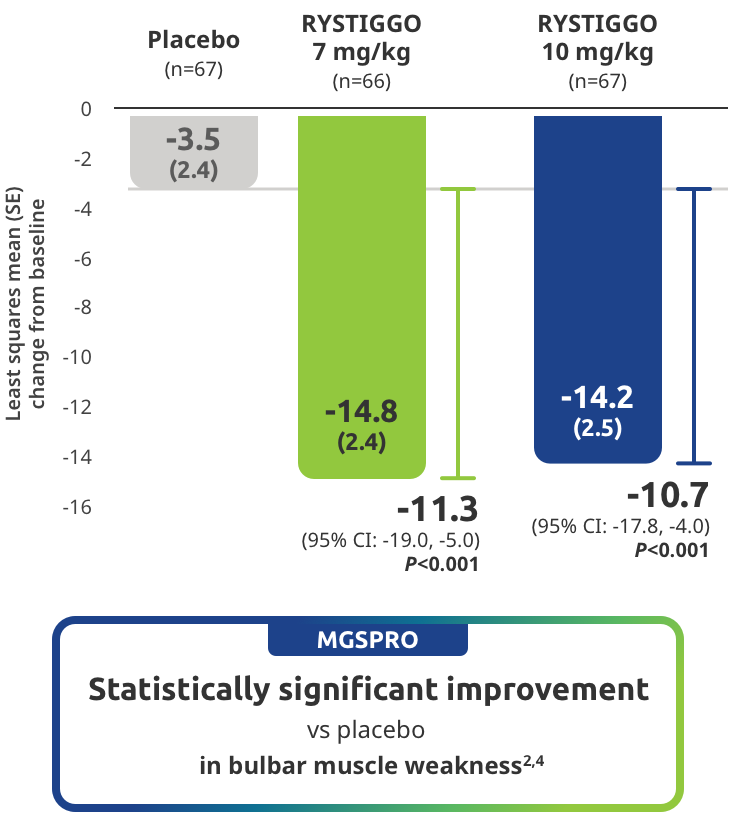
MG Symptoms PRO was part of the planned efficacy analysis; however, efficacy or clinical significance should be interpreted with caution.
MG Symptoms PRO is a novel outcome measure developed by UCB and used in the RYSTIGGO clinical trials, that complements established clinical outcome measures and is the first outcome measure to include a standalone assessment of physical fatigue.2,6,7
Symptoms are measured over a 7-day period. A score ranging from 0-100 is calculated for each domain, with a higher score indicating greater symptom severity.6
3 domains of MG Symptoms PRO were evaluated in the RYSTIGGO pivotal study2,6:
Muscle weakness fatigability: A scale that measures muscle fatigability by assessing use-induced reduction in the ability of the following muscles to function:
- Proximal
- Ocular
- Bulbar
- Respiratory
Physical fatigue: A scale that measures the symptoms/manifestations of physical fatigue, including:
- Body and limb muscle weakness
- Heaviness
- Lack of energy and strength
Bulbar muscle weakness: A scale that measures the symptoms/manifestations associated with bulbar muscle weakness, including:
- Mouth drooping
- Speech and voice problems
- Difficulties with chewing and swallowing
- Facial muscles


Watch a leading expert in gMG review the RYSTIGGO secondary endpoint results
OTHER ENDPOINT
Reduction in immunoglobulin G (IgG) levels1
A reduction in total IgG serum concentrations was observed in adults in both RYSTIGGO treatment groups. Decreases in AChR autoantibody and MuSK autoantibody levels followed a similar pattern.1
Other endpoint: Mean % change from baseline to Week 6 (Day 43) in total IgG level4


§Treatment initiation on Day 1.4
Study limitations: The pharmacological effect of RYSTIGGO was assessed by measuring the decrease in serum IgG levels; the clinical significance of this data has not been established.
Ab+=antibody positive; AChR=acetylcholine receptor; CI=confidence interval; gMG=generalized myasthenia gravis; LS=least squares; MG-ADL=Myasthenia Gravis Activities of Daily Living; MG=myasthenia gravis; MGFA=Myasthenia Gravis Foundation of America; MG Symptoms PRO/MGSPRO=Myasthenia Gravis Symptoms Patient-Reported Outcome; MuSK=muscle-specific tyrosine kinase; SE=standard error.
References:
- RYSTIGGO [Prescribing Information]. Smyrna, GA: UCB, Inc.
- Bril V, Drużdż A, Grosskreutz J, et al. Safety and efficacy of rozanolixizumab in patients with generalised myasthenia gravis (MycarinG): a randomised, double-blind, placebo-controlled, adaptive phase 3 study. Lancet Neurol. 2023;22(5):383-394. doi:10.1016/S1474-4422(23)00077-7
- QMG form. Myasthenia Gravis Foundation of America. 1997. Accessed June 6, 2024. https://myasthenia.org/Portals/0/QMG.pdf
- Data on file. UCB Inc., Smyrna, GA.
- MG composite scale. Myasthenia Gravis Foundation of America. 2012. Accessed June 6, 2024. https://myasthenia.org/Portals/0/MG%20composite%20score.pdf
- Regnault A, Morel T, de la Loge C, et al. Measuring overall severity of myasthenia gravis (MG): evidence for the added value of the MG Symptoms Pro. Neurol Ther. 2023;12(5):1573-1590. doi:10.1007/s40120-023-00464-x
- Cleanthous S, Mork AC, Regnault A, et al. Development of the Myasthenia Gravis (MG) Symptoms PRO: a case study of a patient-centred outcome measure in rare disease. Orphanet J Rare Dis. 2021;16(1):457. doi:10.1186/s13023-021-02064-0
The long-term safety and efficacy of RYSTIGGO for the treatment of adults with anti-AChR Ab+ and anti-MuSK Ab+ gMG was established in two Phase 3 extension studies, MG0004 and MG0007. Extension study results shown here are part of a pooled data analysis.2-4
The primary endpoints in the RYSTIGGO extension studies evaluated the proportion of patients experiencing treatment emergent adverse events (TEAEs). The most common TEAEs (≥10%) reported in adults receiving either dose of RYSTIGGO in the extension studies were headache, diarrhea, COVID-19, decreased blood immunoglobulin G, nausea, and pyrexia.3-5
MG-ADL SCORES IN EXTENSION STUDIES
Improvements were observed in adults receiving subsequent cycles of RYSTIGGO5
Improvements in MG-ADL score across each subsequent 6-week treatment cycle5
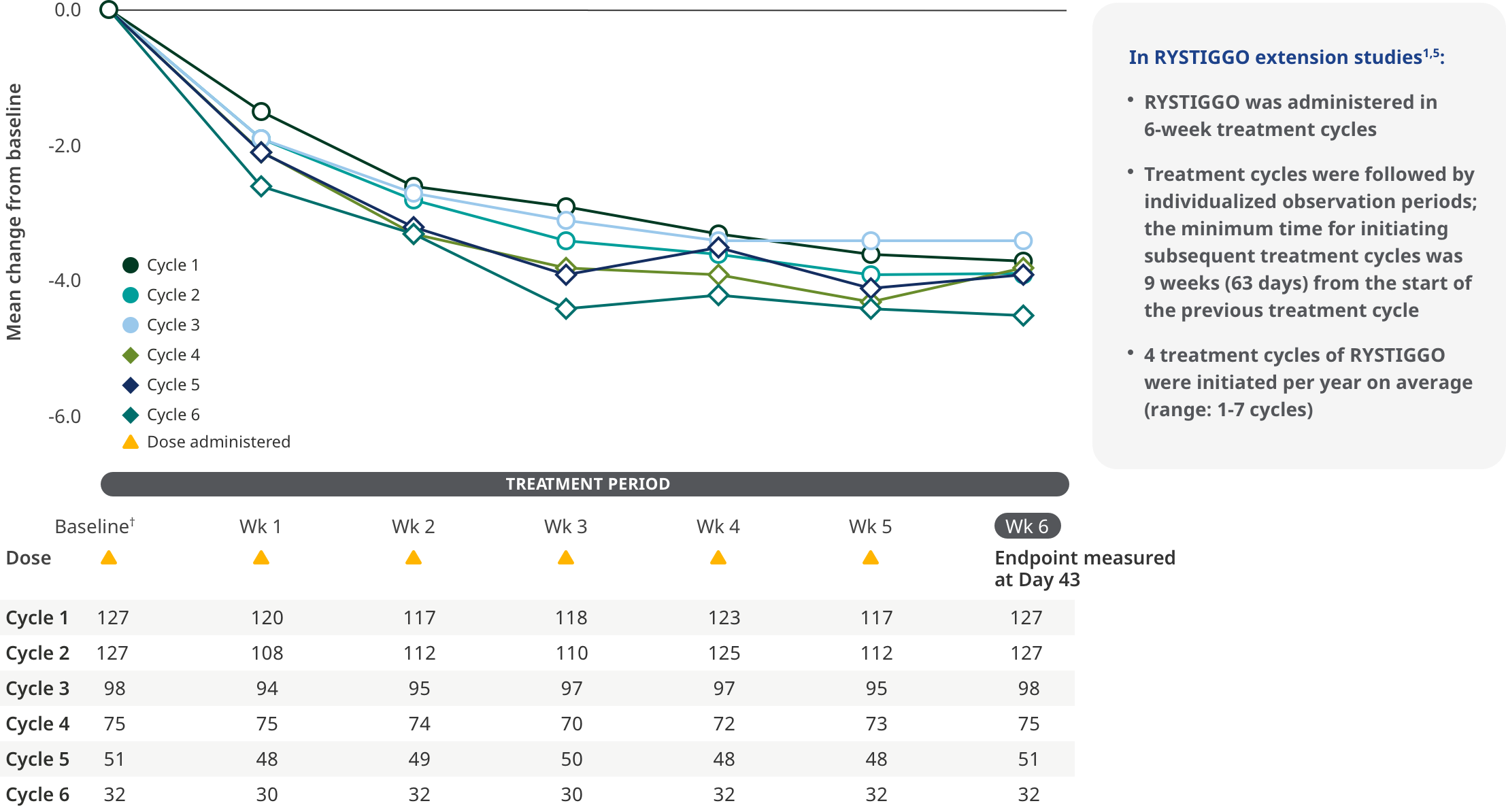
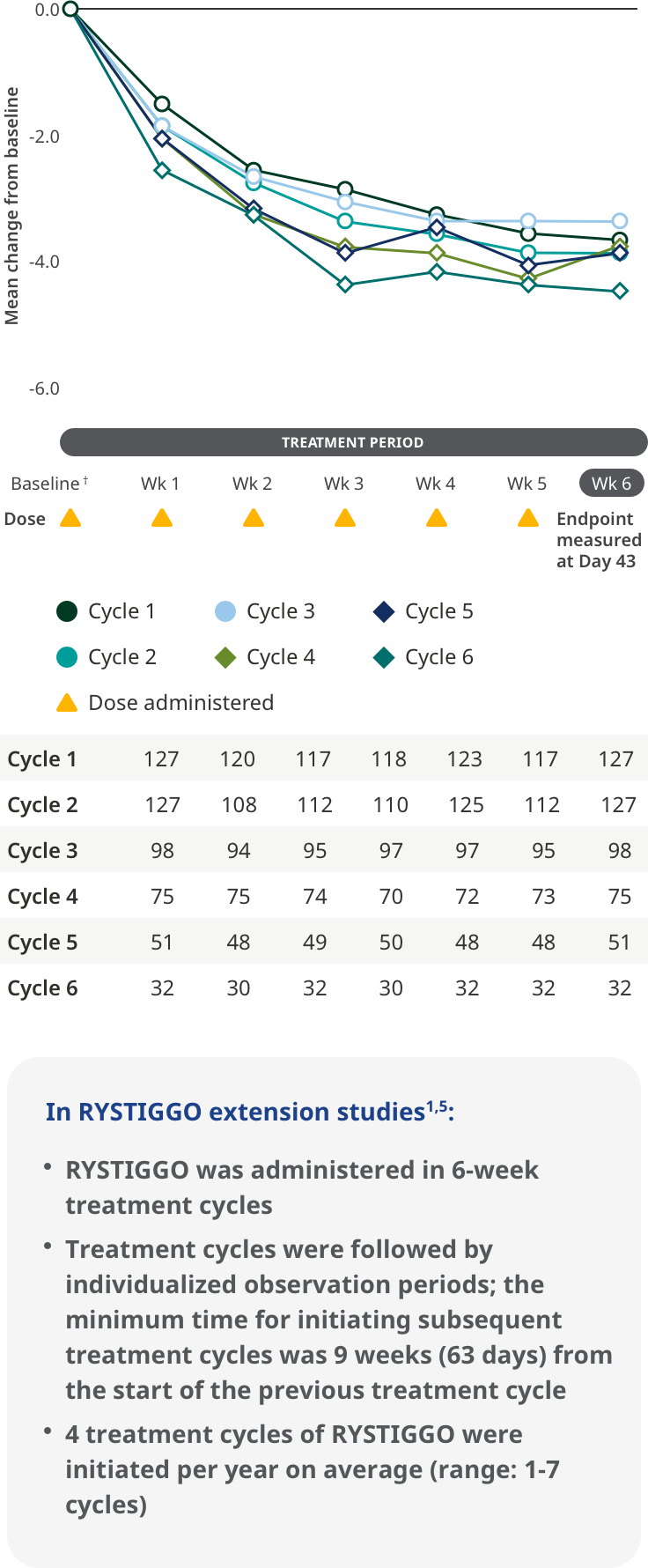
The most common adverse reactions being observed with repeated cyclic treatment were consistent with adverse reactions observed in the pivotal study1,5
†Treatment initiation on Day 1.5
Interim analysis as of July 8, 2022.5
MG-ADL assesses the impact of gMG on daily functions of 8 symptoms on a scale of 0-3, with total scores ranging from 0-24. Higher scores are interpreted as greater impairments. An improvement of ≥2 points was established as clinically meaningful.1,2
Measures include6:
- Voice/speech problems
- Chewing
- Swallowing
- Breathing
- Brushing teeth and/or combing hair
- Rising from a chair
- Diplopia
- Eyelid droop
MINIMAL SYMPTOM EXPRESSION (MSE) IN EXTENSION STUDIES
MSE rates through 6 subsequent cycles of RYSTIGGO
Exploratory endpoint: Proportion of adults achieving MSE with each subsequent cycle of RYSTIGGO5
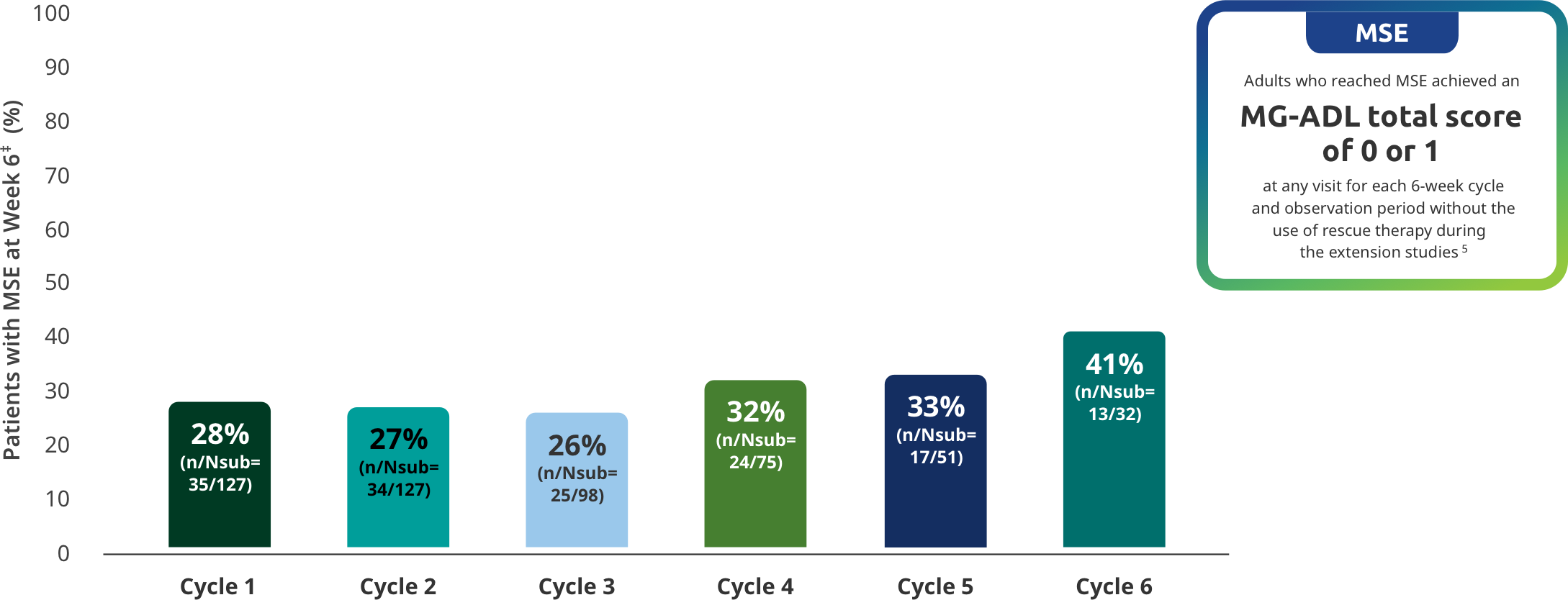

‡Day 43.
MSE was an exploratory endpoint and not controlled for multiplicity. Therefore, data should be interpreted with caution and conclusions cannot be drawn.
Data should be interpreted with caution given the decreasing number of patients receiving subsequent treatment cycles.


Hear the RYSTIGGO extension study results reviewed by an expert in gMG
TREATMENT-FREE INTERVALS
Majority of adults had treatment-free intervals between 4 and 8 weeks5
Time to subsequent symptom-driven treatment cycle from last subcutaneous infusion5
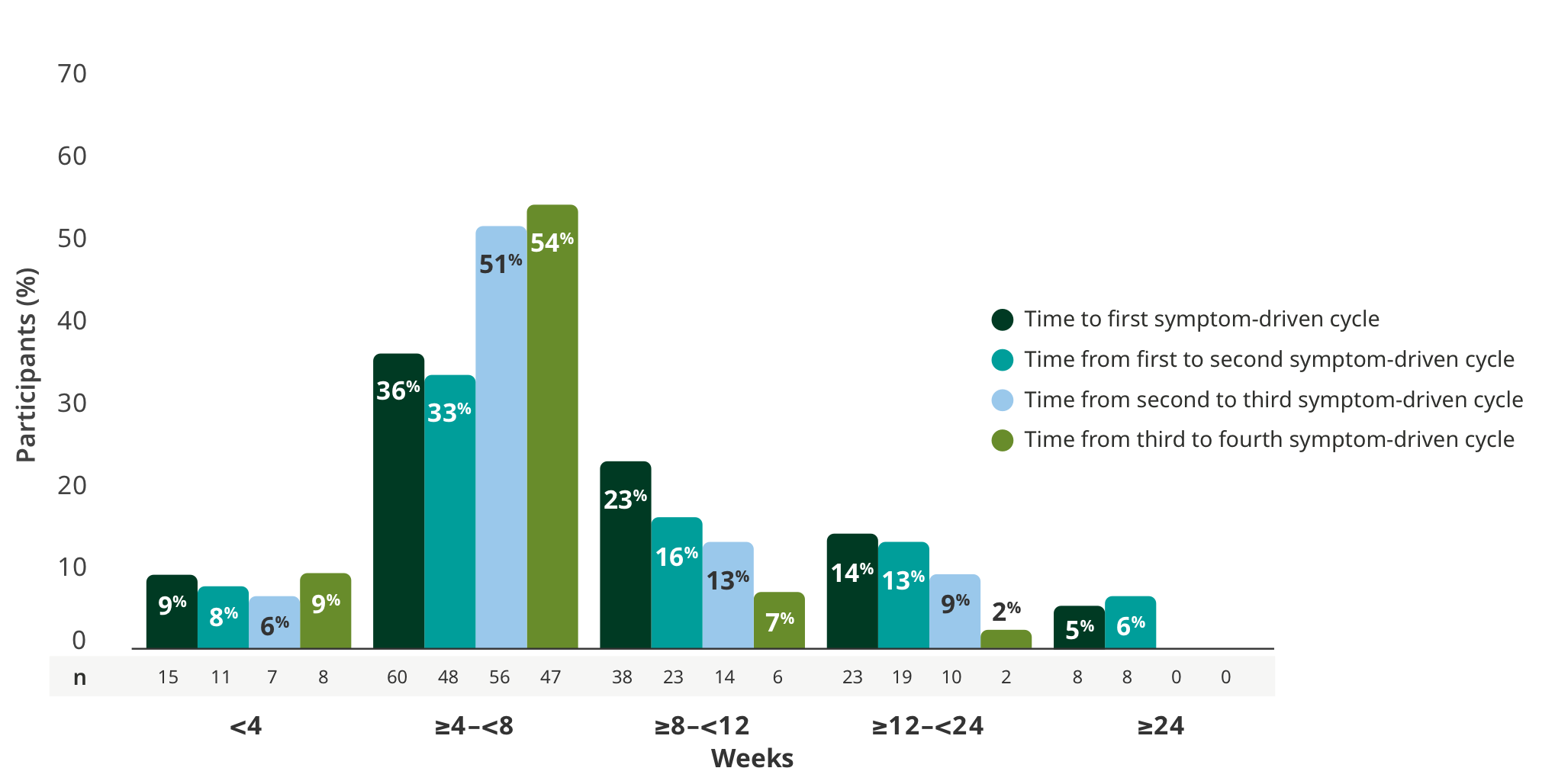
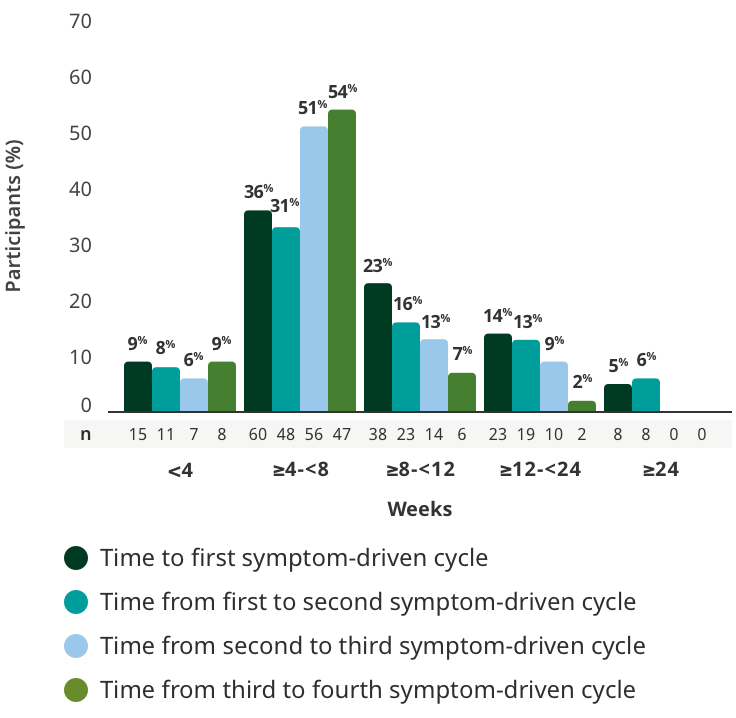
Interim analysis as of July 8, 2022.5
- The median time between treatment cycles was 8 weeks (56 days) for adults treated with RYSTIGGO who initiated 4 cycles1§
- The safety of initiating subsequent cycles sooner than 9 weeks (63 days) from the start of the previous treatment cycle has not been established1
§This is equivalent to 14 weeks (98 days) from the start of the previous treatment cycle.1
Learn more about RYSTIGGO 6-week cyclic dosing
Ab+=antibody positive; AChR=acetylcholine receptor; gMG=generalized myasthenia gravis; MG-ADL=Myasthenia Gravis Activities of Daily Living; MG=myasthenia gravis; MGFA=Myasthenia Gravis Foundation of America; MuSK=muscle-specific tyrosine kinase; Nsub=number of participants with an MSE assessment.
References:
- RYSTIGGO [Prescribing Information]. Smyrna, GA: UCB, Inc.
- Bril V, Drużdż A, Grosskreutz J, et al. Safety and efficacy of rozanolixizumab in patients with generalised myasthenia gravis (MycarinG): a randomised, double-blind, placebo-controlled, adaptive phase 3 study. Lancet Neurol. 2023;22(5):383-394. doi:10.1016/S1474-4422(23)00077-7
- A study to investigate the long-term safety, tolerability, and efficacy of rozanolixizumab in adult patients with generalized myasthenia gravis. ClinicalTrials.gov identifier: NCT04124965. Updated September 5, 2023. Accessed March 8, 2024. https://clinicaltrials.gov/ct2/shaw/NCT04124965?term=MG0004&draw=2&rank=1
- A study to evaluate rozanolixizumab in study participants with generalized myasthenia gravis. ClinicalTrials.gov identifier: NCT04650854. Updated February 26, 2024. Accessed March 8, 2024. https://clinicaltrials.gov/ct2/show/NCT04650854?term=MG0007&draw=2&rank=1
- Data on file. UCB Inc., Smyrna, GA.
- MG activities of daily living (MG-ADL) profile. Myasthenia Gravis Foundation of America. 1997. Accessed May 13, 2024. https://myasthenia.org/Portals/0/ADL.pdf





